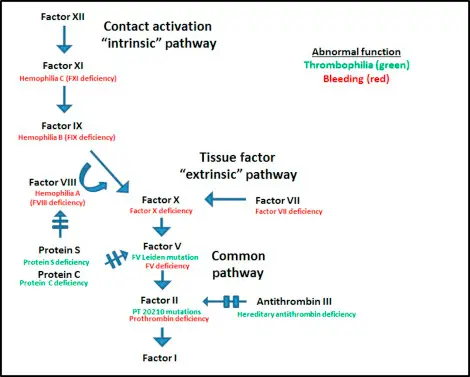
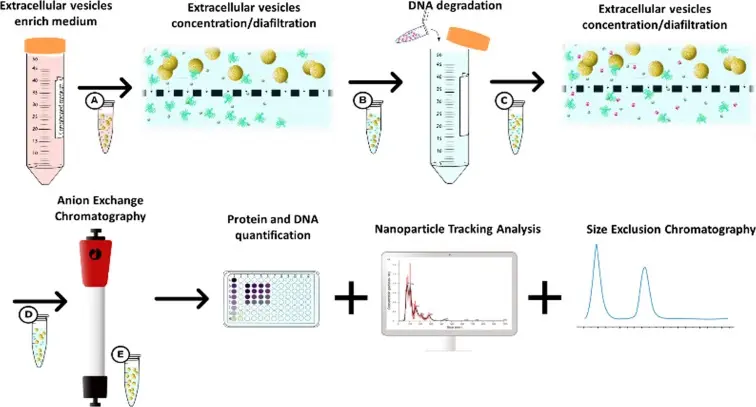
Coagulopathies are a group of disorders that affect the blood’s ability to clot properly, leading to excessive bleeding or clot formation. These conditions, such as haemophilia and von Willebrand disease, affect thousands of patients worldwide, requiring reliable and safe treatments to manage symptoms effectively. Plasma-derived and recombinant medicinal products play a crucial role in these therapies by replenishing missing or defective clotting factors. Since these medicines are administered directly into patients bloodstream, maintaining their absolute safety and purity is of utmost importance. Advanced purification technologies ensure that the medicines are free from contaminants that could cause adverse effects, making safety a top priority in the manufacturing process.
Plasma-Derived and Recombinant Medicinal: Definitions and Differences
Medicinal products used to treat coagulopathies are broadly classified into two types: plasma-derived and recombinant. Plasma-derived products are isolated from donated human blood plasma, which contains natural clotting proteins. However, the availability of plasma and risks of viral contamination are challenges in this approach. Recombinant products, on the other hand, are produced using genetically modified cells such as bacteria or mammalian cell lines in a controlled laboratory environment, eliminating dependence on plasma donations. Recombinant medicines often provide improved consistency and reduced risk of contamination, but their production is complex and costly. Both types have proven clinical efficacy and are integral to modern treatment protocols.
Safety Challenges in Treating Coagulopathies
Producing medicinal products for coagulopathies involves meticulous safety controls. Blood plasma can carry viruses like HIV, hepatitis B and C, and other microbial contaminants. Moreover, impurities such as host cell proteins, residual DNA, and endotoxins from manufacturing processes can affect patient safety. Even trace amounts of these contaminants can trigger immune responses or infections, posing serious health risks. Therefore, regulatory agencies across the world enforce stringent quality standards, requiring manufacturers to implement robust purification methods to ensure that every batch of medicine is safe, pure, and effective before reaching patients.
Purification by Chromatography: A Critical Step
Chromatography is an indispensable technique used to purify plasma-derived and recombinant proteins. This process uses specially designed resins packed inside columns that selectively bind the target proteins, separating them from impurities. Affinity chromatography, in particular, relies on resins that interact specifically with clotting factors or other desired biomolecules. Standard “off-the-shelf” resins are widely used for common proteins, whereas custom-designed resins are developed for unique or complex purification challenges, improving yield and purity. The precision and efficiency of chromatography are vital for producing high-quality medicines that meet regulatory and therapeutic standards.
Role of Prometic Bioseparations
Prometic Bioseparations stands at the forefront of chromatography resin manufacturing, with over 30 years of specialised experience supporting the purification of plasma-derived and recombinant proteins. Their expertise lies in developing both standard and customised resin solutions that meet the complex requirements of biopharmaceutical manufacturers. By enabling effective removal of contaminants and maximising target protein recovery, Prometic’s products help pharmaceutical companies comply with strict global safety standards. This contribution is especially important for producing critical medicines used in treating coagulopathies, where patient safety cannot be compromised.

Innovations and Future Perspectives
The chromatography field is continuously evolving, with innovations focused on creating resins with higher binding capacity, better selectivity, and faster processing times. These advancements allow manufacturers to increase production efficiency while ensuring product safety and purity. Emerging technologies also aim to reduce costs and environmental impact, making therapies more accessible worldwide. Collaborations between companies like Prometic and biopharmaceutical manufacturers are key drivers of these innovations, helping develop next-generation purification solutions tailored to future healthcare needs.